Key Factors Influencing Osseointegration of Dental Implants
Key Factors Influencing Osseointegration of Dental Implants

David CuervoTécnico Especialista en Prótesis Dental en DESS Dental
June 18, 2024

Introduction to Oral Implantology
Oral implantology has transformed dental clinical practices, proving that rehabilitating patients with single, multiple, or complete tooth loss using dental implants is a reliable treatment with a high success rate. The favorable long-term outcomes of oral implant treatments are linked to osseointegration and the positive response of peri-implant tissues.
Essential Elements for Bone Regeneration and Osseointegration
Several crucial factors are necessary for bone regeneration and subsequent osseointegration of a dental implant: cells, clot formation, blood supply, and mechanical stability. Despite meeting these criteria, the total amount of regenerated bone, its long-term stability, and the incidence of complications can vary.
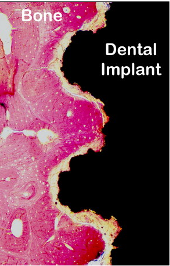
Microscopic histological section of bone embedded in the titanium surface of a dental implant (black)
Enhancing Implant Longevity
Although the longevity of implants is now considered highly reliable, ongoing efforts aim to improve the long-term success of implant rehabilitations and minimise healing complications. This is particularly vital when implant restorations necessitate significant bone regeneration. Factors such as implant surface/design, biomaterials used, surgical technique (submerged vs. non-submerged healing), prosthesis attributes (abutment characteristics, platform changes), and patient-specific factors (systemic health, oral hygiene, compliance) all influence the outcomes.
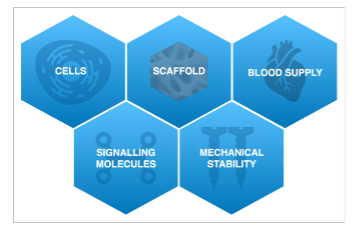
Biological Aspects of Bone Regeneration
Understanding the biological processes during bone regeneration is crucial to grasp how different local and systemic factors impact the regenerative process. Primary healing is essential for successful bone regenerative procedures, with clinician experience, manual dexterity, and patient adherence to pre- and post-operative instructions significantly influencing post-surgical complications.

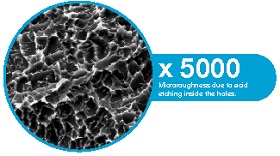
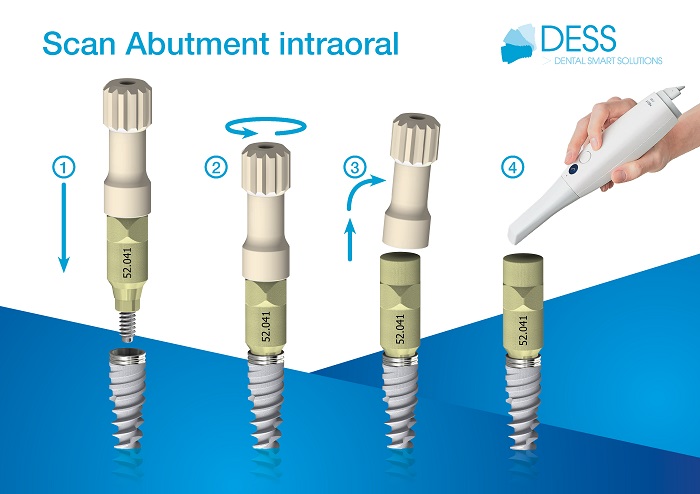
Implant Surface and Bone Regeneration
Analysing clinical evidence shows that implant surface properties can significantly affect peri-implant bone regeneration. Implants vary not only in surface topography but also in design, prosthetic connection, and loading protocol. Proper adjustment of implant surface features could enhance bone regeneration, especially in challenging clinical scenarios.
Impact of Prosthetic Factors
Implant Loading Times
Traditionally, dental implants are prosthetically loaded after a 3 to 6-month healing phase post-implant surgery. To reduce treatment time and accommodate patients, immediate and early prosthetic loading of implants has been introduced. The different implant loading times are:
Immediate Loading
An implant-supported prosthesis is placed on the same day or the day after implant placement.
Early Loading
An implant-supported prosthesis is placed between 1 week and 2 months after implant placement.
Conventional Loading
Implants heal for more than 2 months after placement without connecting a prosthesis.
While immediate and early loading protocols offer quick rehabilitation, careful occlusion consideration is necessary to minimise non-axial forces on the implant. Adequate primary implant stability (30-35 N/cm) is required for immediate loading, which can be challenging in the upper maxilla and in patients needing simultaneous bone regeneration.
Impact of Patient-Related Factors
Risk Factors for Implant Loss
Multiple patient-related factors can affect treatment outcomes: gender, general health status, periodontal status, jaw location, and age at the time of implant placement are all risk factors for implant loss. A patient-centered peri-implant care protocol is recommended, including:
• Updating medical, social, and oral history, risk assessment, and patient feedback.
• Assessing the oral situation: health of peri-implant tissues, prosthetic components, and patient’s oral hygiene ability.
• Controlling risk factors (e.g., smoking, dry mouth, glycemic control).
• Professional intervention with an individualised oral health plan, including oral hygiene training and dentition/implant prophylaxis.
• Determining check-up intervals based on patient, implant, and restoration risk factors.
Systemic Diseases
Osteoporosis
Osteoporosis, associated with reduced bone quality and increased cortical porosity, may affect jawbone quality. Osteoporotic bone should be treated as type IV, and a longer healing period for implant osseointegration is recommended before prosthesis insertion.
Tobacco
Smoking adversely affects wound and bone healing due to local and systemic actions. Nicotine increases platelet adherence, leading to micro clots and ischemia.
Diabetes Mellitus
Diabetes mellitus is linked to complications in the skeletal system, collectively known as “diabetic bone disease” or “diabetic osteopathy.”
Final Considerations for Successful Dental Implant Osseointegration
While patient-related factors are not absolute contraindications for implant-associated bone regeneration, they influence treatment success and complication risks. Therefore, selecting patients carefully, managing underlying medical conditions and associated risk factors, and consulting with the patient’s physician when necessary is recommended.

David Cuervo
Técnico Especialista en Prótesis Dental en DESS Dental